LUTATHERA slowed NET progression for longer in people with recently diagnosed, faster-growing NETs
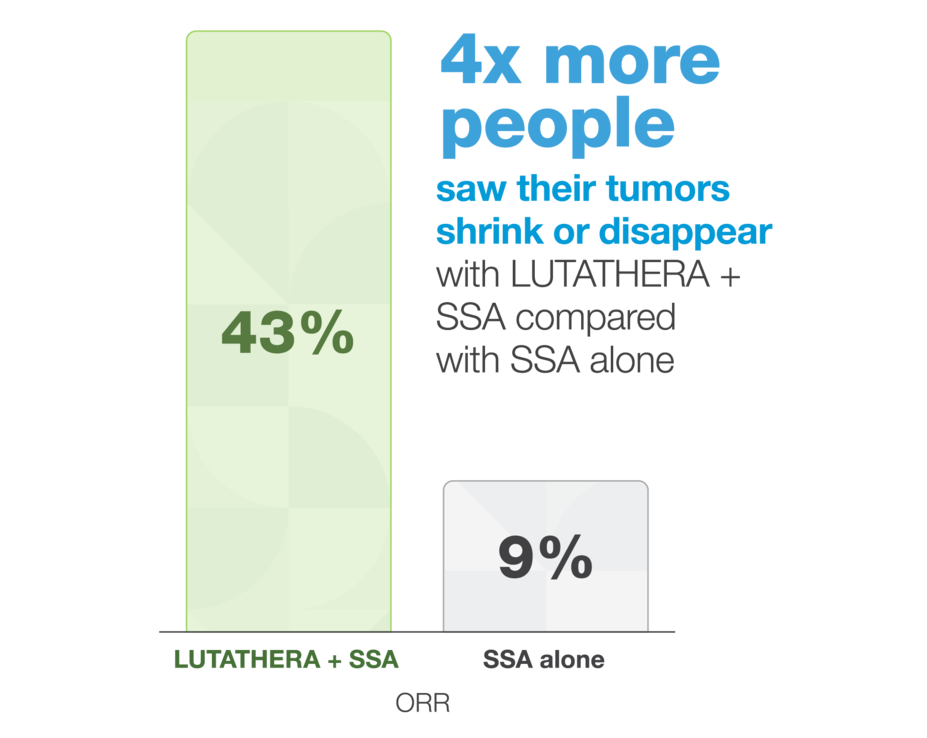
The NETTER-2 trial included 226 people who were recently diagnosed with SSTR+ GEP-NETs that were faster growing (Ki-67, 10%–55%). They were split into 2 groups: 151 received LUTATHERA and a long-acting SSA, and 75 received a long-acting, high-dose SSA alone.
The study measured progression-free survival (PFS). PFS is the amount of time cancer doesn’t grow or spread during and after treatment. Median PFS is the length of time when half of the people treated have not yet progressed. It’s about slowing progression.
LUTATHERA works with SSA to give people more time without progression
Tumors were more likely to shrink with LUTATHERA
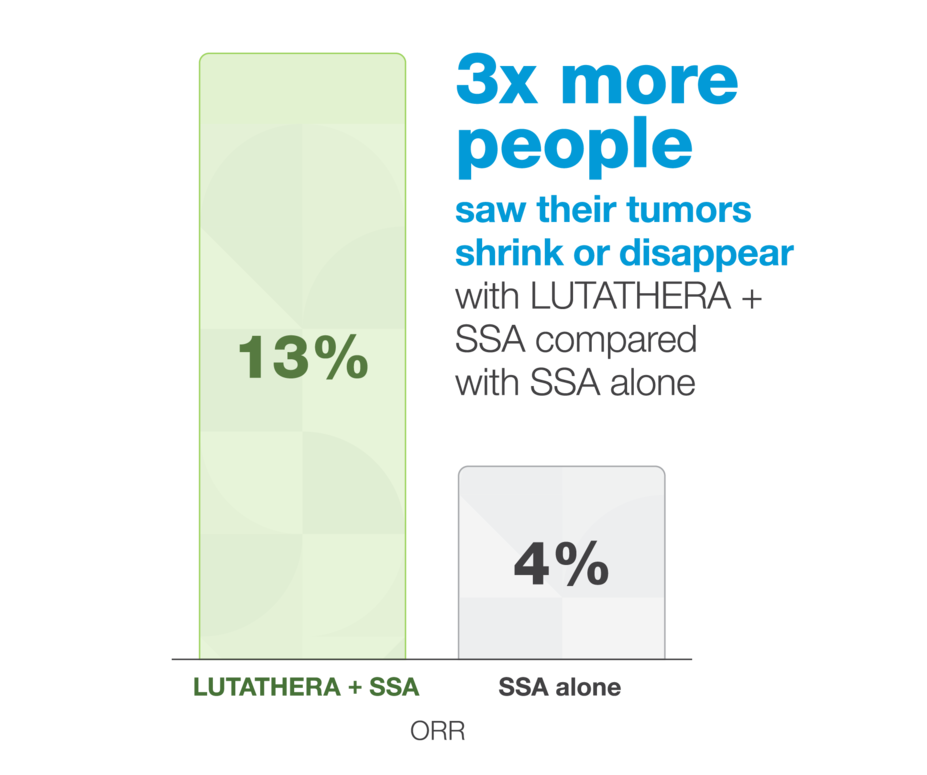
The study also measured objective response rate (ORR). ORR is the percentage of people whose cancer got smaller or disappeared.
LUTATHERA slowed NET progression for longer in people with NETs that progressed on SSA
The NETTER-1 trial included 229 people with SSTR+ GEP-NETs who had tumors that were slower growing (Ki-67, 0%–20%) and progressed on treatment with an SSA. They were split into 2 groups: 116 received LUTATHERA and a long-acting SSA, and 113 received a long-acting, high-dose SSA alone.
LUTATHERA works with SSA to give people more time without progression
In people taking LUTATHERA + SSA, more than half were progression free at a 14-month check-in. In people taking SSA alone, half had their disease progress at 8.5 months.
Tumors were more likely to shrink with LUTATHERA
Watch a real patient's journey with LUTATHERA
Meet Donna and Don, who share their story of self-advocacy and the power of family
Since her diagnosis, Donna and her husband Don's lives have been changed by NETs. Listen as they tell their story of self-advocacy and strength rooted in the love of their family. See how Donna took control over her NET journey with LUTATHERA.
GEP-NET, gastroenteropancreatic neuroendocrine tumor; NET, neuroendocrine tumor; SSA, somatostatin analogue; SSTR+, somatostatin receptor-positive.